Cover Picture
Borosilicates as deep-ultraviolet transparent nonlinear optical crystals: structural motifs, performance limits and future directions
Yangfeifei Ou, Xiao-Liang Zhou, You-Zhao Lan, Jian-Wen Cheng* Submit a Manuscript
Borosilicates as deep-ultraviolet transparent nonlinear optical crystals: structural motifs, performance limits and future directions
Yangfeifei Ou, Xiao-Liang Zhou, You-Zhao Lan, Jian-Wen Cheng* Submit a Manuscript
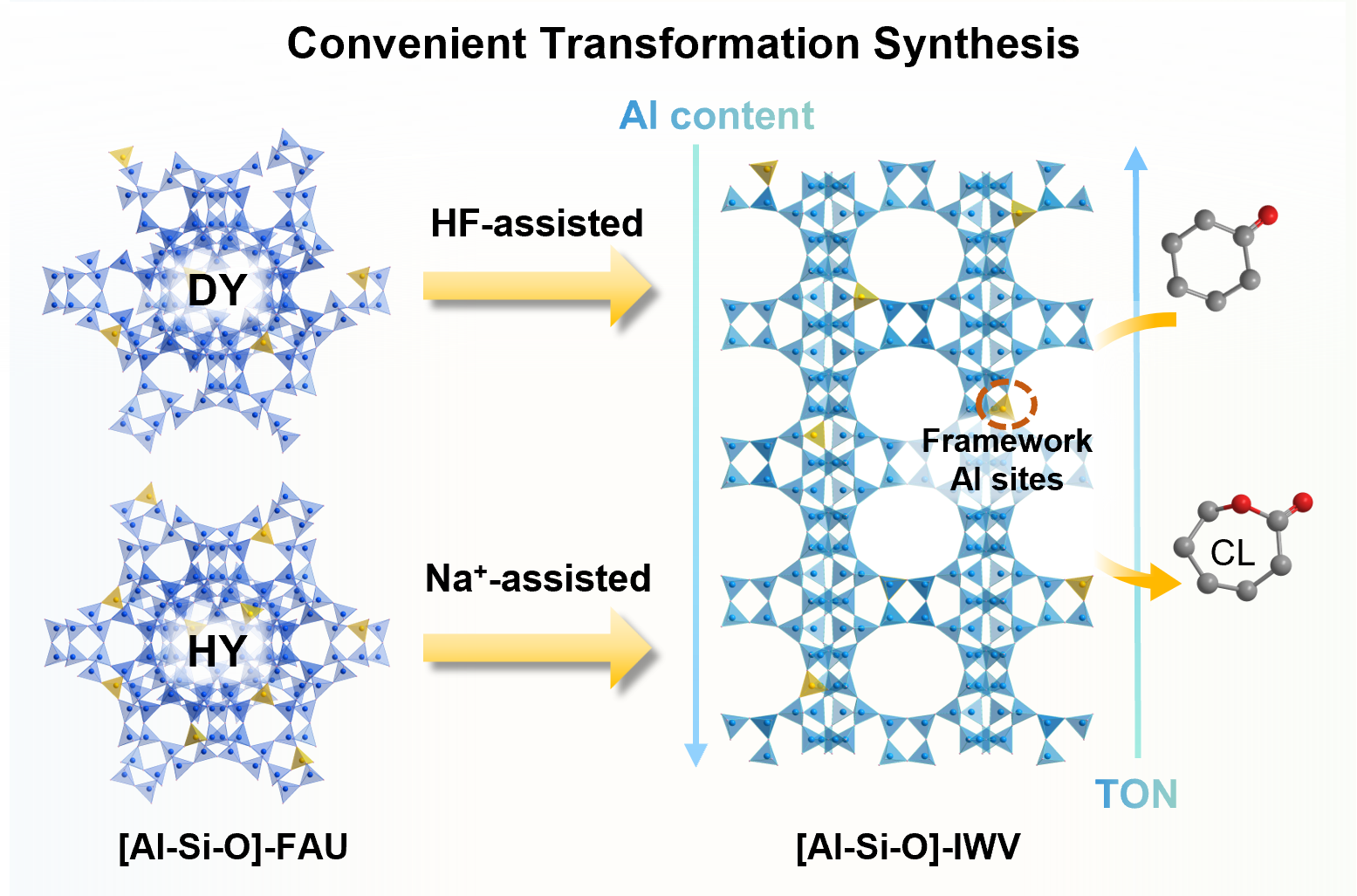
Convenient transformation of FAU zeolites to IWV aluminosilicates with adjustable Al sites for Baeyer-Villiger oxidation
Meichen Jiao, Sitong Liu, Chunmin Jia, Hao Xu, Zhiguo Zhu, Peng Wu*, and Xian-Ming Zhang*
Chin. J. Struct. Chem., 2025, 44(9), 100654. DOI: 10.1016/j.cjsc.2025.100654
September 1, 2025
Interzeolite transformation; IWV aluminosilicates; Aluminum content; Baeyer-Villiger oxidation; ε-caprolactone
ABSTRACT
The Baeyer–Villiger (BV) oxidation of cyclohexanone was explored using IWV-type aluminosilicates with different Al sites as heterogeneous catalysts. The IWV framework exhibits a two-dimensional 12-membered ring (MR) pore system that is intersected by 14-MR supercages, resembling typical beta zeolite. To address the constraints associated with hydrothermal synthesis, IWV aluminosilicates were synthesized via interzeolite transformation of various FAU-type zeolites. HF-assisted transformation of dealuminated FAU zeolite resulted in the formation of a high-silica IWV aluminosilicate (Si/Al = 54.6), whereas the incorporation of aluminum isopropoxide enabled the tuning of the Si/Al ratio down to 18.7. The alkaline conversion of protonated FAU zeolites, utilizing Na+ ions as mineralizing agents, produced high-Al content IWV derivatives in just four days. Catalytic evaluation demonstrated that the high-silica IWV catalyst exhibited a higher turnover number than the other IWV catalysts, along with enhanced ε-caprolactone (CL) selectivity relative to that of high-silica beta zeolite. Facile modifications were performed to adjust Al sites, as characterized by pyridine-adsorbed infrared spectroscopy. Experimental evidence confirmed that Al Brønsted acid sites improved the selective oxidation of cyclohexanone, while concurrently enhancing CL hydrolysis.