Just Accepted
Just Accepted Articles have been posted online after technical editing and typesetting for immediate view. The final edited version with page numbers will appear in the Current Issue soon.
Submit a Manuscript

Enhancing the photocatalytic activity of crystalline g-C3N4 towards NO oxidation and CO2 reduction through K+-doping and cyano defect engineering
Zhou Li, Mengxue Yu, Shixin Chang, Zhibin Huang, Zhenmin Cheng, Weibin Zhang, Sónia A.C. Carabineiro, Zhigao Xu, Kangle Lv*
https://doi.org/10.1016/j.cjsc.2025.100698
ABSTRACT
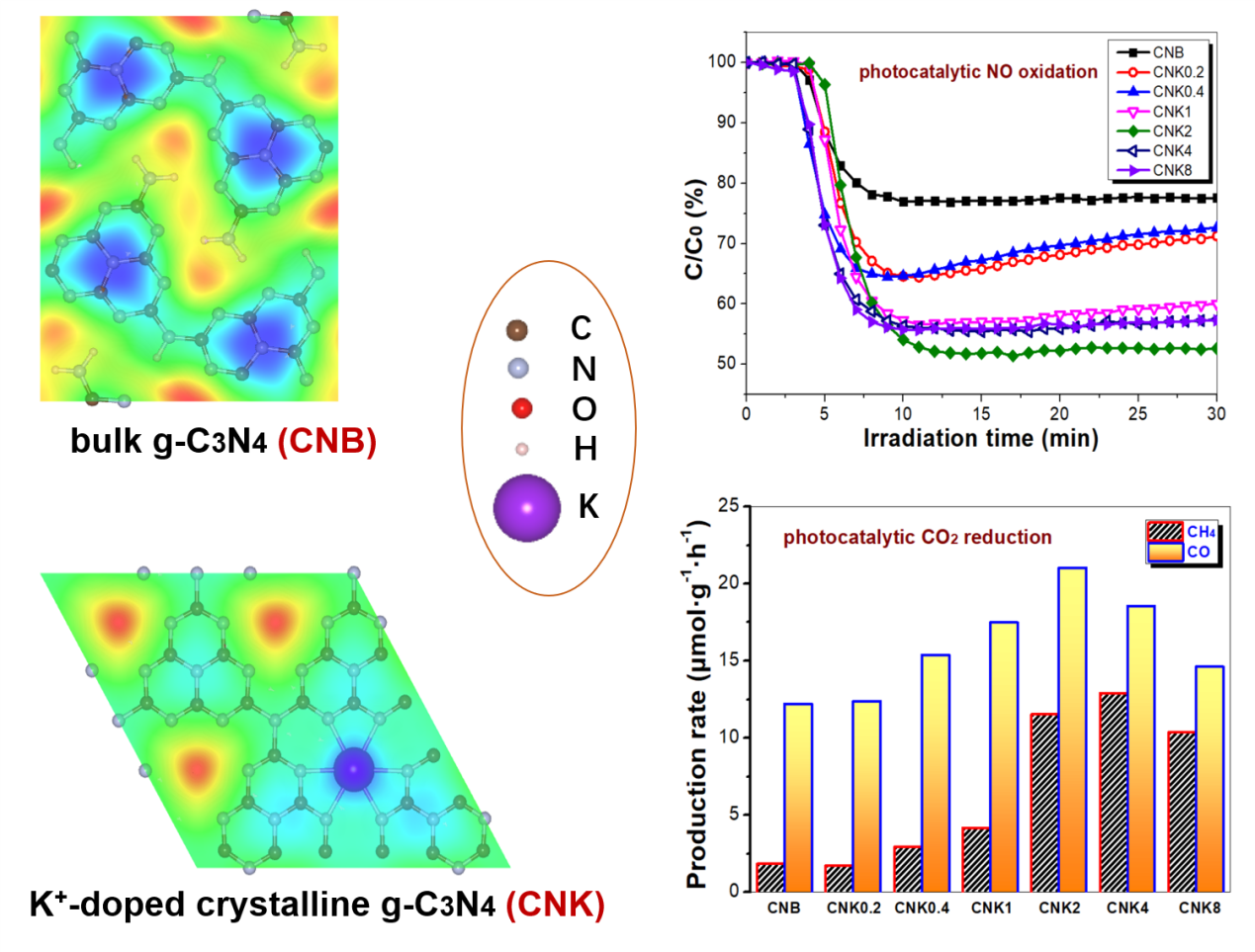
The polymeric semiconductor photocatalyst graphitic carbon nitride (g-C3N4) has attracted considerable attention due to its visible-light responsiveness and excellent biocompatibility. However, the photocatalytic efficiency of bulk g-C3N4 (CNB) remains insufficient for pratical applications, primarily due to its limited light absorption range and the rapid charge carrier recombination. In this study, K+-doped crystalline g-C3N4 with cyano defects (CNK) was synthesized by the calcination of dicyandiamide in the presence of KCl. The addition of KCl promoted the formation of K+-doped crystalline g-C3N4 with cyano defects. The optimized photocatalyst (CNK2) exhibited the highest photocatalytic activity for NO oxidation, achieving a removal rate of 47.40%, which is 2.1 times higher than that of CNB. This enhancement is mainly attributed to the increased generation of reactive oxygen species (ROS), particularly superoxide radicals (•O2-) and singlet oxygen (1O2). Furthermore, improved performance in photocatalytic CO2-to-CH4 conversion was also observed, which is attributed to the formation of a build-in electric field (BIEF) induced by K+ ion doping and the introduction of cyano defects.