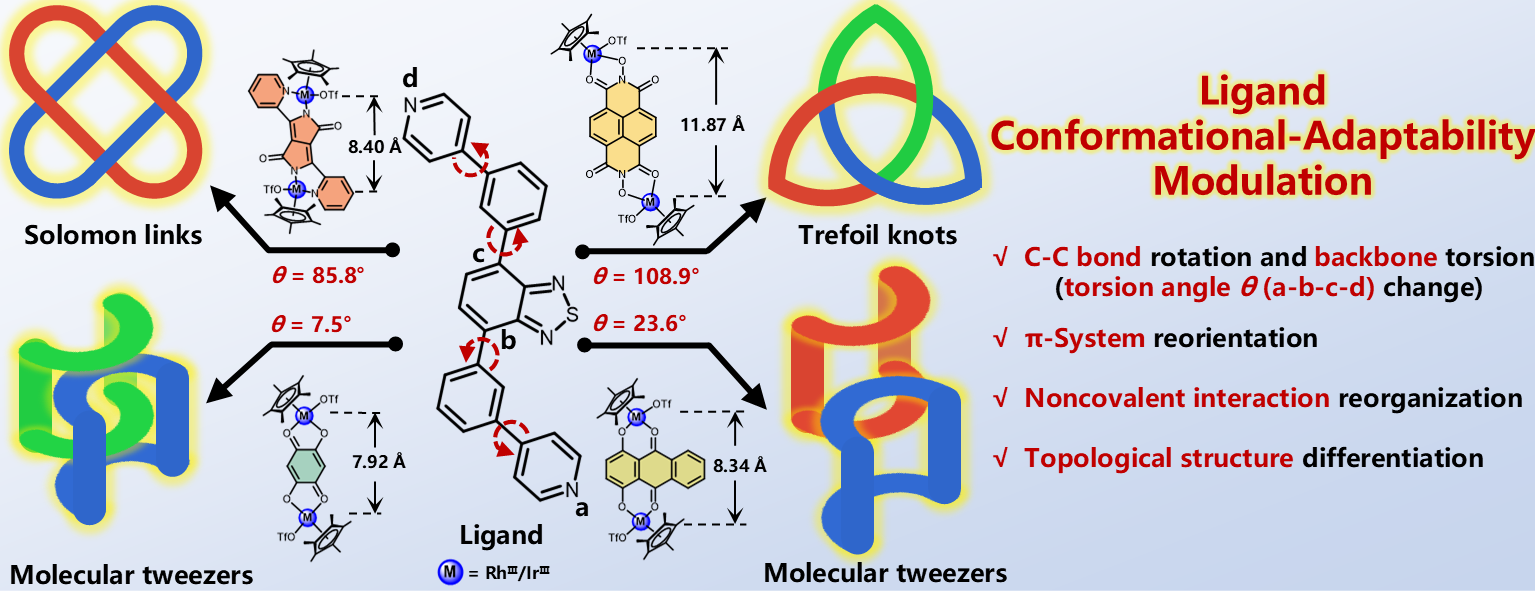
Ligand conformational adaptability modulated self-assembly of Solomon links (412 ) and trefoil knots (31)
Xing-Cheng Hu, Qiu-Shui Mu, Shu-Jin Bao, Yan Zou, Xin-Yu Wang, Guo-Xin Jin*
Chin. J. Struct. Chem., 2025, 44(10), 100712. DOI: 10.1016/j.cjsc.2025.100712
October 15, 2025
Coordination-driven self-assembly; Half-sandwich units; Solomon links; Trefoil knots; Conformational adaptability
ABSTRACT
Mechanically interlocked molecules (MIMs) have unique properties with broad applications, yet constructing both knotted and linked topologies from the same ligand remains challenging due to their distinct geometric demands. To address this, we design and synthesize a conformationally adaptive ligand 4,7-bis(3-(pyridin-4-yl)phenyl) benzo[c][1,2,5]thiadiazole (L1) with a tunable torsional angle θ of N1–C1–C2–N2 ranging from 7.5° to 108.9°. Utilizing coordination-driven self-assembly at ambient temperature, L1 selectively assembles with binuclear half-sandwich units Rh-B1, Rh-B2, Rh-B3, and Rh-B4 featuring Cp*RhⅢ (Cp* = η5-pentamethylcyclopentadienyl) into distinct topologies: Solomon links Rh-1, trefoil knots Rh-2, molecular tweezers Rh-3, and Rh-4, respectively. Crucially, the self-adaptability of ligand L1 directs topology formation through programming different combination of noncovalent interactions (π−π stacking, CH⋯π interaction, and lone pair-π interaction), thus navigating divergent assembly pathways by conformational switching, as evidenced by X-ray crystallography analysis, independent gradient model (IGM) analysis, detailed nuclear magnetic resonance (NMR) spectroscopy and electrospray ionization time-of-flight/mass spectrometry (ESI-TOF/MS). This strategy can also be extended to construct Cp*IrⅢ analogs (Solomon links Ir-1, trefoil knots Ir-2, molecular tweezers Ir-3 and Ir-4), demonstrating metal-independent control and achieving intricate topologies in a high yield.